Coupled Plasma Mass Spectrometry (ICP-MS) is a cornerstone in modern analytical chemistry, offering unparalleled capabilities in elemental and isotopic analysis. Though its intricate machinery and scientific terminology might seem daunting, unravelling its essential concepts is vital for beginners keen on harnessing its analytical prowess.
This in-depth guide aims to navigate the complex landscape of fundamentals of ICP-MS by breaking down five fundamental pillars that constitute its core. Mastering these foundations allows you to embark on an enlightening journey through precise elemental quantification and isotopic investigation.
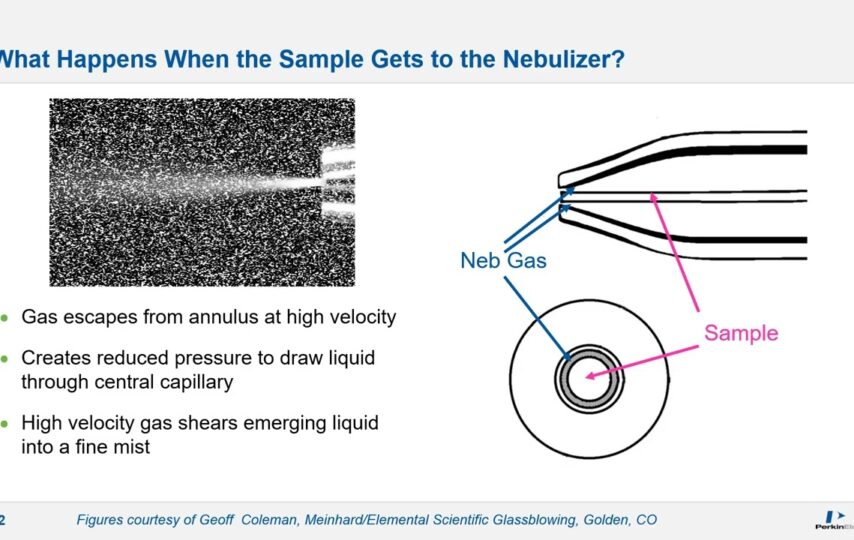
Introduction to ICP-MS: Where Fusion Meets Precision
ICP-MS amalgamates two formidable techniques: inductively coupled plasma and mass spectrometry. The journey begins with igniting a high-temperature inductively coupled plasma—an extraordinary scientific feat resembling a controlled star on Earth.
Like the sun’s surface, this plasma’s searing heat swiftly vaporises the sample material, transforming its atomic constituents into ions. These ions, bearing the elemental information of the sample, are meticulously transported into the heart of the mass spectrometer for critical analysis.
ICP-MS uniquely marries the ability to break apart complex samples through the plasma’s heat with the precision of mass spectrometry’s mass-to-charge ratio separation. This harmonious partnership allows for identifying elements in the selection and their quantification, a pivotal capability in various scientific endeavours.
Understanding Ionization: The Metamorphosis of Matter
At the heart of ICP-MS lies the astonishing process of ionisation, a kind of elemental magic. Within the scorching embrace of the inductively coupled plasma, atoms undergo a radical transformation.
The intense heat strips away their outermost electrons, creating electrically charged particles known as ions. These ions, now bearing a positive charge, carry the distinct elemental signature of their origin.
This metamorphic shift is a scientific marvel and the starting point of our analytical journey. These charged ions become the messengers of elemental insight, guided precisely through the apparatus. As they navigate magnetic fields and energy gradients, their masses are decoded, revealing the sample’s elemental composition.
Mass Separation in Mass Spectrometry: Unveiling the Puzzle
At the heart of mass spectrometry lies the intricate art of mass separation—a process that disentangles ions based on their mass-to-charge ratios. In ICP-MS, a mass analyser acts as a discerning gatekeeper, allowing only specific ions to pass through its gates while deflecting others.
Different mass analysers, such as quadrupoles, magnetic sectors, and time-of-flight analysers, facilitate this separation with varying resolution and sensitivity. By separating ions based on their masses, the mass analyser enables the identification and quantification of elements present in the sample.
Detection and Quantification: Navigating the Subatomic
Realm ICP-MS’s analytical prowess lies in its ability to detect a wide range of elements and its exceptional sensitivity. Its dynamic range spans from parts per billion (ppb) to parts per trillion (ppt). It is an indispensable tool for applications demanding high precision and accuracy, such as environmental monitoring, geology, and pharmaceutical research.
Achieving accurate quantification involves careful calibration using known standards that span the concentration range of interest. Additionally, internal measures of a known concentration of an element not typically found in the sample are employed to correct for variations in instrument performance.
Through meticulous calibration and internal standardisation, ICP-MS ensures that the detected signal correlates accurately with the concentration of elements in the sample.
5. Dealing with Interferences: The Web of Analytical Challenges
While ICP-MS excels in providing accurate elemental information, it’s not immune to analytical challenges. Interferences can arise from a phenomenon known as isobaric overlap, where different elements or isotopes share the same mass-to-charge ratio. This overlap can lead to misidentification and inaccurate quantification.
To combat interferences, researchers employ ingenious strategies. Collision/reaction cells, for example, introduce a controlled collision environment that alters the behaviour of ions, effectively mitigating the interference effect.
On the other hand, high-resolution mass analysers resolve isobaric overlaps by distinguishing slight mass differences that may not have been distinguishable with lower-resolution instruments.
Conclusion
Cultivating Analytical Mastery As you delve into the intricate world of ICP-MS, these five fundamental concepts will serve as your guiding stars. The fusion of inductively coupled plasma and mass spectrometry, the transformation of atoms into ions, the art of mass separation, the precision of quantification, and the strategy to overcome interferences collectively shape your understanding of this remarkable analytical technique.
Your journey with ICP-MS is not just an exploration of scientific boundaries. It’s an immersion into the beauty of elemental analysis, the precision of mass separation, and the intellectual thrill of solving complex analytical puzzles. Armed with this foundational knowledge, you’re poised to venture into advanced applications, confident in your ability to unravel the mysteries of elemental composition with unmatched accuracy and insight.